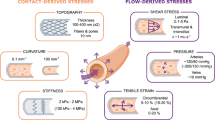
Abstract
The shape and morphology of endothelial cells (ECs) lining the blood vessels are a good indicator for atheroprone and atheroprotected sites. ECs of blood vessels experience both wall shear stress (WSS) and cyclic stretch (CS). These mechanical stimuli influence the shape and morphology of ECs. A few models have been proposed for predicting the morphology of ECs under WSS or CS. In the present study, a mathematical cell population model is developed to simulate the morphology of ECs under combined WSS and CS conditions. The model considers the cytoskeletal filaments, cell–cell interactions, and cell–extracellular matrix interactions. In addition, the reorientation and polymerization of microfilaments are implemented in the model. The simulations are performed for different conditions: without mechanical stimuli, under pure WSS, under pure CS, and under combined WSS and CS. The results are represented as shape and morphology of ECs, shape index, and angle of orientation. The model is validated qualitatively and quantitatively with several experimental studies, and good agreement with experimental studies is achieved. To the best of our knowledge, it is the first model for predicting the morphology of ECs under combined WSS and CS condition. The model can be used to indicate the atheroprone regions of a patient’s artery.

















Similar content being viewed by others
References
Barbee KA, Mundel T, Lal R, Davies PF (1995) Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am J Physiol Heart Circ Physiol 268:H1765–H1772
Bicknell R (1996) Endothelial cell culture. Cambridge University Press, Cambridge
Breen LT, McHugh PE, Murphy BP (2009) Multi-axial mechanical stimulation of HUVECs demonstrates that combined loading is not equivalent to the superposition of individual wall shear stress and tensile hoop stress components. J Biomech Eng 131:081001–081001
Coskun H, Li Y, Mackey MA (2007) Ameboid cell motility: a model and inverse problem, with an application to live cell imaging data. J Theor Biol 244:169–179
Cucina A et al (1995) Shear stress induces changes in the morphology and cytoskeleton organisation of arterial endothelial cells. Eur J Vasc Endovasc Surg 9:86–92
Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519
Dejana E (2004) Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Foucard L, Vernerey FJ (2012a) Dynamics of stress fibers turnover in contractile cells. J Eng Mech 138:1282–1287
Foucard L, Vernerey FJ (2012b) A thermodynamical model for stress–fiber organization in contractile cells. Appl. Phys. Lett. 100:013702
Franke R-P, Gräfe M, Schnittler H, Seiffge D, Mittermayer C, Drenckhahn D (1984) Induction of human vascular endothelial stress fibres by fluid shear stress. Nature 307:648–649
Freitas RA (1999) Nanomedicine, volume I: basic capabilities. Landes Bioscience, Georgetown
Galbraith C, Skalak R, Chien S (1998) Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton 40:317–330
Gatland IR (1994) Numerical integration of Newton’s equations including velocity-dependent forces. Am J Phys 62:259–265
Gracheva ME, Othmer HG (2004) A continuum model of motility in ameboid cells. Bull Math Biol 66:167–193
Green J, Waters S, Shakesheff K, Byrne H (2009) A mathematical model of liver cell aggregation in vitro. Bull Math Biol 71:906–930
Haghighipour N, Tafazzoli-Shadpour M, Shokrgozar MA, Amini S (2010) Effects of cyclic stretch waveform on endothelial cell morphology using fractal analysis. Artif Organs 34:481–490
Hayakawa K, Sato N, Obinata T (2001) Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp Cell Res 268:104–114
Helmlinger G, Geiger R, Schreck S, Nerem R (1991) Effects of pulsatile flow on cultured vascular endothelial cell morphology. J Biomech Eng 113:123–131
Hsu H-J, Lee C-F, Kaunas R (2009) A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One 4:e4853
Hsu H-J, Lee C-F, Locke A, Vanderzyl SQ, Kaunas R (2010) Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One 5:e12470
Jackson TL, Byrne HM (2002) A mechanical model of tumor encapsulation and transcapsular spread. Math Biosci 180:307–328
Jamali Y, Azimi M, Mofrad MR (2010) A sub-cellular viscoelastic model for cell population mechanics. PLoS One 5:e12097
Jarvis RA (1973) On the identification of the convex hull of a finite set of points in the plane. Inf Process Lett 2:18–21
Kang J, Steward RL, Kim Y, Schwartz RS, LeDuc PR, Puskar KM (2011) Response of an actin filament network model under cyclic stretching through a coarse grained Monte Carlo approach. J Theor Biol 274:109–119
Kaunas R, Hsu H-J (2009) A kinematic model of stretch-induced stress fiber turnover and reorientation. J Theor Biol 257:320–330
Kaunas R, Nguyen P, Usami S, Chien S (2005) Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci USA 102:15895–15900
Lee C-F, Haase C, Deguchi S, Kaunas R (2010) Cyclic stretch-induced stress fiber dynamics-Dependence on strain rate. Rho-kinase MLCK Biochem Biophys Res Commun 401:344–349
Levesque M, Nerem R (1985) The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng 107:341–347
Levesque M, Sprague E, Schwartz C, Nerem R (1989) The influence of shear stress on cultured vascular endothelial cells: the stress response of an anchorage-dependent mammalian cell. Biotechnol Prog 5:1–8
Li S, Huang NF, Hsu S (2005) Mechanotransduction in endothelial cell migration. J Cell Biochem 96:1110–1126
Lodish H (2008) Molecular cell biology. Macmillan, London
Lu L, Feng Y, Hucker WJ, Oswald SJ, Longmore GD, Yin FCP (2008) Actin stress fiber pre-extension in human aortic endothelial cells. Cell Motil Cytoskeleton 65:281–294
Maurin B, Cañadas P, Baudriller H, Montcourrier P, Bettache N (2008) Mechanical model of cytoskeleton structuration during cell adhesion and spreading. J Biomech 41:2036–2041
Melchior B, Frangos JA (2010) Shear-induced endothelial cell–cell junction inclination. Am J Physiol Cell Physiol 299:C621–C629
Moore JE Jr, Bürki E, Suciu A, Zhao S, Burnier M, Brunner HR, Meister J-J (1994) A device for subjecting vascular endothelial cells to both fluid shear stress and circumferential cyclic stretch. Ann Biomed Eng 22:416–422
Mott RE, Helmke BP (2007) Mapping the dynamics of shear stress-induced structural changes in endothelial cells. Am J Physiol Cell Physiol 293:C1616–C1626
Nerem RM, Levesque MJ, Cornhill J (1981) Vascular endothelial morphology as an indicator of the pattern of blood flow. J Biomech Eng 103:172
Noria S, Xu F, McCue S, Jones M, Gotlieb AI, Langille BL (2004) Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. Am J Pathol 164:1211–1223
Ohashi T, Sato M (2005) Remodeling of vascular endothelial cells exposed to fluid shear stress: experimental and numerical approach. Fluid dynamics research 37:40–59
Ohashi T, Sugawara H, Matsumoto T, Sato M (2000) Surface topography measurement and intracellular stress analysis of cultured endothelial cells exposed to fluid shear stress. JSME Int J Ser C Mech Syst Mach Elem Manuf 43:780–786
Ookawa K, Sato M, Ohshima N (1992) Changes in the microstructure of cultured porcine aortic endothelial cells in the early stage after applying a fluid-imposed shear stress. J Biomech 25:1321–1328
Osborn EA, Rabodzey A, Dewey CF, Hartwig JH (2006) Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am J Physiol Cell Physiol 290:C444–C452
Pakravan HA, Saidi MS, Firoozabadi B (2015) FSI simulation of a healthy coronary bifurcation for studying the mechanical stimuli of endothelial cells under different physiological conditions. J Mech Med Biol 1550089
Reneman RS, Arts T, Hoeks AP (2006) Wall shear stress—an important determinant of endothelial cell function and structure-in the arterial system in vivo. J Vasc Res 43:251–269
Sato M, Levesque MJ, Nerem RM (1987) Micropipette aspiration of cultured bovine aortic endothelial cells exposed to shear stress. Atertio Thromb Vasc Biol 7:276–286
Stamenović D, Lazopoulos KA, Pirentis A, Suki B (2009) Mechanical stability determines stress fiber and focal adhesion orientation. Cell Mol Bioeng 2:475–485
Thoumine O, Ziegler T, Girard PR, Nerem RM (1995) Elongation of confluent endothelial cells in culture: the importance of fields of force in the associated alterations of their cytoskeletal structure. Exp Cell Res 219:427–441
Wang JH-C, Goldschmidt-Clermont P, Wille J, Yin FC-P (2001) Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech 34:1563–1572
Yamada H, Takemasa T, Yamaguchi T (2000) Theoretical study of intracellular stress fiber orientation under cyclic deformation. J Biomech 33:1501–1505
Yamaguchi T, Yamamoto Y, Liu H (2000) Computational mechanical model studies on the spontaneous emergent morphogenesis of the cultured endothelial cells. J Biomech 33:115–126
Zeng Y, Yip AK, Teo S-K, Chiam K-H (2012) A three-dimensional random network model of the cytoskeleton and its role in mechanotransduction and nucleus deformation. Biomech Model Mechanobiol 11:49–59
Zhao S, Suciu A, Ziegler T, Moore JE, Bürki E, Meister J-J, Brunner HR (1995) Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Atertio Thromb Vasc Biol 15:1781–1786
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pakravan, H.A., Saidi, M.S. & Firoozabadi, B. A mechanical model for morphological response of endothelial cells under combined wall shear stress and cyclic stretch loadings. Biomech Model Mechanobiol 15, 1229–1243 (2016). https://doi.org/10.1007/s10237-015-0756-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0756-z